Understanding receptor-mediated endocytosis of elastic nanoparticles through coarse grained molecular dynamic simulation†
Abstract
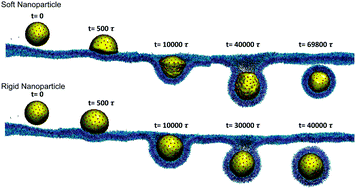
For nanoparticle (NP)-based drug delivery platforms, the elasticity of the NPs has a significant influence on their blood circulation time and cellular uptake efficiency. However, due to the complexity of the endocytosis process and the inconsistency in the definition of elasticity for NPs in experiments, the understanding about the receptor-mediated endocytosis process of elastic NPs is still limited. In this work, we developed a coarse-grained molecular dynamics (CGMD) model for elastic NPs. The energy change of the elastic NPs can be precisely controlled by the bond, area, volume and bending potentials of this CGMD model. To represent liposomes with different elasticities, we systematically varied the bending rigidity of elastic NPs in CGMD simulations. Additionally, we changed the radius of the elastic NPs to explore the potential size effect. Through virtual nano-indentation tests, we found that the effective stiffness of elastic NPs was determined by their bending rigidity and size. Afterwards, we investigated the receptor-mediated endocytosis process of elastic NPs with different sizes and bending rigidities. We found that the membrane wrapping of soft NPs was faster than that of the stiff ones at the early stage, due to the NP deformation induced large contact area between the NPs and the membrane. However, because of the large energy penalties induced by the NP deformation, the membrane wrapping speed of soft NPs slows down during the late stage. Eventually, the soft NPs are wrapped less efficiently than the stiff ones during the membrane wrapping process. Through systematic CGMD simulations, we found a scaling law between the cellular uptake efficiency and the phenomenal bending rigidity of elastic NPs, which agrees reasonably well with experimental observations. Furthermore, we observed that the membrane wrapping efficiencies of soft and stiff NPs with large sizes were close to each other, due to the stronger ligand–receptor binding force and smaller difference in the stiffness of elastic NPs. Our computational model provides an effective tool to investigate the receptor-mediated endocytosis of elastic NPs with well controlled mechanical properties. This study can also be applied to guide the design of NP-based drug carriers with high efficacy, by utilizing their elastic properties.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...