On the crystallisation temperature of very high-density amorphous ice
Abstract
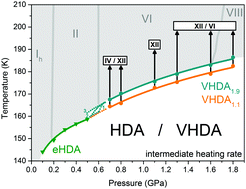
The influence of the protocol of preparation on the crystallisation temperature TX of very high-density amorphous ice (VHDA) was studied by varying the annealing pressure (1.1, 1.6 and 1.9 GPa) and temperature (160, 167 and 175 K, respectively). TX increases by up to 4 K in the pressure range of 0.7 to 1.8 GPa for samples annealed at 1.9 GPa compared to samples annealed at 1.1 GPa. Concomitantly, secondary crystallisation channels are suppressed, indicating the absence of structural inhomogeneities. For VHDA prepared at 1.1 GPa and 1.6 GPa our results indicate such inhomogeneities, which we regard to be incompletely amorphized, distorted nanodomains of hexagonal ice that cannot be detected through X-ray diffraction experiments. VHDA prepared at high pressures and temperatures thus represents the amorphous state of water at >0.7 GPa least affected by nanocrystals that has been described so far. We expect the TX obtained for the samples prepared in this manner to be close to the ultimate limit, i.e., we do not consider it possible to raise the low-temperature border to the no-man's land notably further by changing the preparation protocol. An additional, considerable increase in this border will only be possible by working at much shorter time-scales, e.g., by employing fast heating experiments.


 Please wait while we load your content...
Please wait while we load your content...