Experimental evidence of TICT state in 4-piperidinyl-1,8-naphthalimide – a kinetic and mechanistic study
Abstract
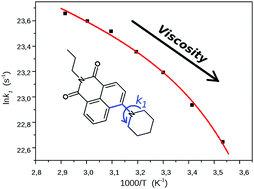
The excited state processes in N-propyl-4-piperidinyl-1,8-naphthalimide have been studied by measuring its fluorescence spectra and decay curves in solvents of different polarity and viscosity and also in a frozen solvent glass. The results unanimously proved the formation of a dark twisted intramolecular charge transfer (TICT) state from the emissive charge transfer (CT) species, the direct product of excitation. The rate coefficients of the TICT process and the deactivations of the CT and TICT species were determined, using a reversible two-state kinetic model. The temperature dependence of the kinetic data was consistent with a kinetic barrier consisting of three terms, the inherent barrier of the reaction, and the contributions of the solute–solvent interactions related to the solvent viscosity and polarity. The potential energy surfaces were calculated in the S0 and the S1 states along the coordinate of turning motion which was conclusive concerning the direction of the twisting and indicated a possible conformational change of the piperidinyl unit. The theoretical calculations confirmed that the TICT species is dark and has a stronger charge transfer character compared to the CT state.


 Please wait while we load your content...
Please wait while we load your content...