Theoretical kinetic study of the formic acid catalyzed Criegee intermediate isomerization: multistructural anharmonicity and atmospheric implications†
Abstract
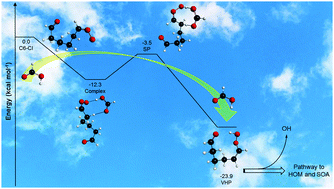
We performed a theoretical study on the double hydrogen shift isomerization reaction of a six carbon atom Criegee intermediate (C6-CI), catalyzed by formic acid (HCOOH), to produce vinylhydroperoxide (VHP), C6-CI + HCOOH → VHP + HCOOH. This Criegee intermediate can serve as a surrogate for larger CIs derived from important volatile organic compounds like monoterpenes, whose reactivity is not well understood and which are difficult to handle computationally. The reactant HCOOH exerts a pronounced catalytic effect on the studied reaction by lowering the barrier height, but the kinetic enhancement is hindered by the multistructural anharmonicity. First, the rigid ring-structure adopted by the saddle point to facilitate simultaneous transfer of two atoms does not allow the formation of as many conformers as those formed by the reactant C6-CI. And second, the flexible carbon chain of C6-CI facilitates the formation of stabilizing intramolecular C–H⋯O hydrogen bonds; this stabilizing effect is less pronounced in the saddle point structure due to its tightness and steric effects. Thus, the contribution of the reactant C6-CI conformers to the multistructural partition function is larger than that of the saddle point conformers. The resulting low multistructural anharmonicity factor partially cancels out the catalytic effect of the carboxylic acid, yielding in a moderately large rate coefficient, k(298 K) = 4.9 × 10−13 cm3 molecule−1 s−1. We show that carboxylic acids may promote the conversion of stabilized Criegee intermediates into vinylhydroperoxides in the atmosphere, which generates OH radicals and leads to secondary organic aerosols, thereby affecting the oxidative capacity of the atmosphere and ultimately the climate.
- This article is part of the themed collection: Bunsentagung 2018: Kinetics in the Real World


 Please wait while we load your content...
Please wait while we load your content...