A theoretical study on mixtures of amino acid-based ionic liquids†
Abstract
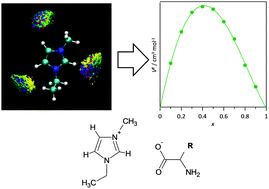
Ionic liquid mixtures containing amino acid anions are studied at the microscopic level using molecular dynamics simulations. The analysis of relevant features such as intermolecular forces (hydrogen bonding), molecular level arrangements, and properties of solvation spheres, allowed inferring the structuring of the studied mixtures. The effects of mixture compositions and the number of cations and anions were analysed in detail. The reported results showed even distribution of anions around cations. The absence of microheterogeneities and low deviations from ideality are due to the similar mechanism of interaction between the considered anions and cations. Likewise, the most relevant features are produced by the development of hydrogen bonding between the amino acid carboxylate group and hydrogen bond donor sites in the cations.


 Please wait while we load your content...
Please wait while we load your content...