A DFT+U study of the catalytic degradation of 1,2-dichloroethane over CeO2†
Abstract
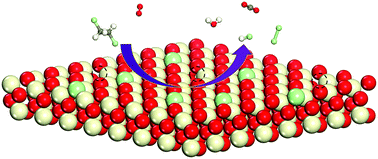
The catalytic degradation of 1,2-dichloroethane (DCE) at CeO2(111) was investigated using periodic density functional theory calculations corrected by on-site Coulomb interactions. From thorough calculations of possible elementary steps, we are able to identify the lowest energy reaction pathway for the catalytic oxidation of DCE at CeO2(111). It proceeds via two successive C–Cl bond breaking processes to form adsorbed CH2CH2 species, and after further dehydrogenation and C–C bond scission, the surface species are finally oxidized to CO2 and H2O. The surface oxygen vacancies were found to be important for the catalytic decomposition of DCE, by providing the adsorption sites, as well as for charge transfer to favor C–Cl bond breaking. We are also able to illustrate the effect of H2O on the catalytic activity of CeO2(111) for DCE oxidation.


 Please wait while we load your content...
Please wait while we load your content...