Triarylmethyl-based 2D covalent networks: virtual screening of chemical functionalisation for optimising strain-induced property control†
Abstract
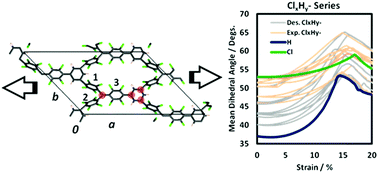
Two-dimensional covalent networks based on triarylmethyl (TAM) radical monomers have been proposed as versatile materials whose unpaired electrons may be externally localised/delocalised through the application of external uniaxial strain. This phenomenon arises through the strain-induced variance of the dihedral twist angles of the aryl rings within the network, and allows the control of important physico-chemical properties (e.g. magnetic interactions, electronic band gap). In order to experimentally realise such materials, one must find a compromise between the kinetic stability of the TAM monomers (through sterically protecting the radical centre with the appropriate aryl ring functionalisation) and the structural flexibility of the resulting material (provided by low intra-ring steric hindrance). In this work, through an efficient search procedure based on force field-based screening, employing ∼1750 calculations, followed by selected accurate electronic structure calculations, we provide support for the experimental viability of TAM-based 2D networks with highly controllable properties.


 Please wait while we load your content...
Please wait while we load your content...