X-ray photochemistry of carbon hydride molecular ions†
Abstract
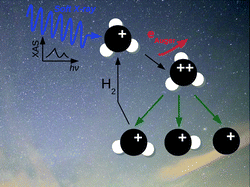
Hydride molecular ions are key ingredients of the interstellar chemistry since they are precursors of more complex molecules. In regions located near a soft X-ray source these ions may resonantly absorb an X-ray photon which triggers a complex chain of reactions. In this work, we simulate ab initio the X-ray absorption spectrum, Auger decay processes and the subsequent fragmentation dynamics of two hydride molecular ions, namely CH2+ and CH3+. We show that these ions feature strong X-ray absorption resonances which relax through Auger decay within 7 fs. The doubly-charged ions thus formed mostly dissociate into smaller ionic carbon fragments: in the case of CH2+, the dominant products are either C+/H+/H or CH+/H+. For CH3+, the system breaks primary into CH2+ and H+, which provides a new route to form CH2+ near a X-ray source. Furthermore, our simulations provide the branching ratios of the final products formed after the X-ray absorption as well as their kinetic and internal energy distributions. Such data can be used in the chemistry models of the interstellar medium.


 Please wait while we load your content...
Please wait while we load your content...