Bromine adatom promoted C–H bond activation in terminal alkynes at room temperature on Ag(111)†
Abstract
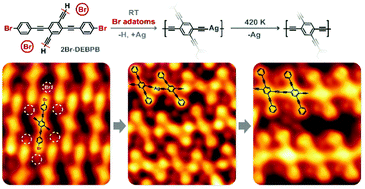
The activation of C–H bonds in terminal alkynyl groups at room temperature was achieved in the reaction of 2,5-diethynyl-1,4-bis(4-bromophenylethynyl)benzene on Ag(111). Scanning tunneling microscopy studies showed the formation of organometallic species, whose stabilization was confirmed by density functional theory calculations, at room temperature as the product of C–H bond activation. The partial conversion of organometallic structures into covalent products of the homocoupling between the terminal alkynes was achieved by further annealing the sample at 420 K. Detached Br adatoms were suggested to play a key role in promoting the C–H bond activation. This proposal was supported by the theoretical study based on a simplified model of the system, showing the weakening of the C–H bond in the alkynyl group by an approaching Br atom. The results provide a new strategy for on-surface C–H bond activation under mild conditions, which register great potential applications in on-surface synthesis and bottom-up preparation of functional nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...