Noble gas encapsulated B40 cage†
Abstract
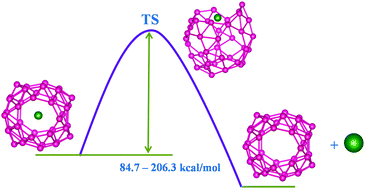
The efficacy of B40 borospherene to act as a host for noble gas atoms is explored via density functional theory based computations. Although the Ng@B40 complexes are thermochemically unstable with respect to dissociation into free Ng and B40, it does not rule out their viability as all the systems possess a high activation free energy barrier (84.7–206.3 kcal mol−1). Therefore, once they are formed, it is hard to take out the Ng atom. Two Ng atoms can also be incorporated within B40 for the lighter Ng atoms (He and Ne). In fact, the destabilization offered by the encapsulation of one and two He atoms and one Ne atom inside B40 is significantly less than that in experimentally synthesized He@C20H20, highlighting their greater possibility for synthesis. Although Ar2 and Kr2 encapsulated B40 systems are very much destabilized by the repulsive interaction between Ng2 and B40, an inspection of the bonding situation reveals that the confinement can even induce some degree of covalent interaction between two otherwise non-bonded Ng atoms. Ng atoms transfer electrons towards B40 which is smaller for lighter Ng atoms and gradually increases along He to Rn. Even if the electrostatic interaction between Ng and B40 is the most predominant term in these systems, the extent of the orbital interaction is also considerable. However, the very large Pauli repulsion counterbalances the attractive interaction, eventually turning the interaction repulsive in nature. Ng@B40 also shows dynamical behaviour involving continuous exchange between hexagonal and heptagonal holes, similar to the host cage, as understood from the very little variation in the activation barrier because of the Ng encapsulation. Furthermore, sandwich complexes like [(η5-C5Me5)Fe(η6-B40)]+ and [(η5-C5Me5)Fe(η7-B40)]+ are noted to be viable with the latter being slightly more stable than the former. The encapsulation of Xe slightly improves the dissociation energy associated with the decomposition into Xe@B40 and [Fe(η5-C5Me5)]+ compared to that in the bare one.


 Please wait while we load your content...
Please wait while we load your content...