Enthalpic interactions in aqueous strong electrolytes upon addition of ionic liquids†
Abstract
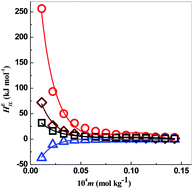
The present study deals with the inter-ionic interactions between strong electrolytes and ionic liquids based on the thermodynamic properties such as excess partial molar enthalpy, HEIL, relative apparent molar enthalpy, ϕL, and the enthalpic interaction parameters. The thermodynamic properties of the systems are the key indicators to understand the interionic interactions. We have conducted a systematic investigation of the enthalpic behavior of aqueous solution of salts and ionic liquids and their mixtures. The present study also emphasizes how the HEIL values for the mixture of aqueous solution of ionic liquids and salts deviate from linearity as compared to those of the constituent aqueous ionic liquid or salt. This deviation from linearity for the HEIL values has been discussed here.


 Please wait while we load your content...
Please wait while we load your content...