Exploration of bulk and interface behavior of gas molecules and 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid using equilibrium and nonequilibrium molecular dynamics simulation and quantum chemical calculation
Abstract
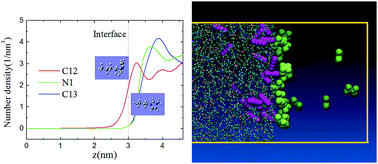
Ionic liquids (ILs) show brilliant performance in separating gas impurities, but few researchers have performed an in-depth exploration of the bulk and interface behavior of penetrants and ILs thoroughly. In this research, we have performed a study on both molecular dynamics (MD) simulation and quantum chemical (QC) calculation to explore the transport of acetylene and ethylene in the bulk and interface regions of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM]–[BF4]). The diffusivity, solubility and permeability of gas molecules in the bulk were researched with MD simulation first. The subdiffusion behavior of gas molecules is induced by coupling between the motion of gas molecules and the ions, and the relaxation processes of the ions after the disturbance caused by gas molecules. Then, QC calculation was performed to explore the optical geometry of ions, ion pairs and complexes of ions and penetrants, and interaction potential for pairs and complexes. Finally, nonequilibrium MD simulation was performed to explore the interface structure and properties of the IL–gas system and gas molecule behavior in the interface region. The research results may be used in the design of IL separation media.


 Please wait while we load your content...
Please wait while we load your content...