Understanding the reaction mechanism of the oxidative addition of ammonia by (PXP)Ir(i) complexes: the role of the X group†
Abstract
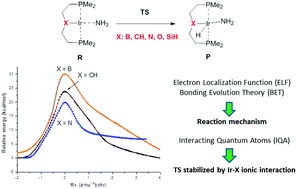
An analysis of the electronic rearrangements for the oxidative addition of ammonia to a set of five representative (PXP)Ir pincer complexes (X = B, CH, O, N, SiH) is performed. We aim to understand the factors controlling the activation and reaction energies of this process by combining different theoretical strategies based on DFT calculations. Interestingly, complexes featuring higher activation barriers yield more exothermic reactions. The analysis of the reaction path using the bonding evolution theory shows that the main chemical events, N–H bond cleavage and Ir–H bond formation, take place before the transition structure is reached. Metal oxidation implies an electron density transfer from non-shared Ir pairs to the Ir–N bond. This decrement in the atomic charge of the metal provokes different effects in the ionic contribution of the Ir–X bonding depending on the nature of the X atom as shown by the interacting quantum atoms methodology.


 Please wait while we load your content...
Please wait while we load your content...