A high pressure study of calmodulin–ligand interactions using small-angle X-ray and elastic incoherent neutron scattering†
Abstract
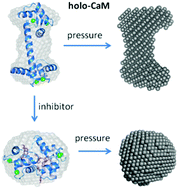
Calmodulin (CaM) is a Ca2+ sensor and mediates Ca2+ signaling through binding of numerous target ligands. The binding of ligands by Ca2+-saturated CaM (holo-CaM) is governed by attractive hydrophobic and electrostatic interactions that are weakened under high pressure in aqueous solutions. Moreover, the potential formation of void volumes upon ligand binding creates a further source of pressure sensitivity. Hence, high pressure is a suitable thermodynamic variable to probe protein–ligand interactions. In this study, we compare the binding of two different ligands to holo-CaM as a function of pressure by using X-ray and neutron scattering techniques. The two ligands are the farnesylated hypervariable region (HVR) of the K-Ras4B protein, which is a natural binding partner of holo-CaM, and the antagonist trifluoperazine (TFP), which is known to inhibit holo-CaM activity. From small-angle X-ray scattering experiments performed up to 3000 bar, we observe a pressure-induced partial unfolding of the free holo-CaM in the absence of ligands, where the two lobes of the dumbbell-shaped protein are slightly swelled. In contrast, upon binding TFP, holo-CaM forms a closed globular conformation, which is pressure stable at least up to 3000 bar. The HVR of K-Ras4B shows a different binding behavior, and the data suggest the dissociation of the holo-CaM/HVR complex under high pressure, probably due to a less dense protein contact of the HVR as compared to TFP. The elastic incoherent neutron scattering experiments corroborate these findings. Below 2000 bar, pressure induces enhanced atomic fluctuations in both holo-CaM/ligand complexes, but those of the holo-CaM/HVR complex seem to be larger. Thus, the inhibition of holo-CaM by TFP is supported by a low-volume ligand binding, albeit this is not associated with a rigidification of the complex structure on the sub-ns Å-scale.


 Please wait while we load your content...
Please wait while we load your content...