N-Doped graphene-supported PdCu nanoalloy as efficient catalyst for reducing Cr(vi) by formic acid†
Abstract
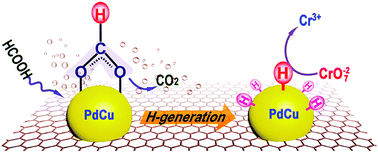
Reducing Cr(VI) to Cr(III) with formic acid is desirable for environmental protection, but the sluggish kinetics limits its practical application, which currently motivates the intensive study of efficient catalysts for this redox reaction. Here bimetallic PdCu nanoalloy (∼5 nm in size) supported by N-doped graphene was synthesized through a one-pot hydrothermal process. The catalytic activity of PdCu nanoalloy highly depends on the Pd/Cu atomic ratio and N-doped graphene support. The obtained Pd6Cu4/NG shows superior catalysis towards the Cr(VI) reduction by formic acid with a high kinetic constant (kn = 23.2 min−1 mg−1) and a low activation energy (Ea = 34.9 kJ mol−1). Active H atoms were found to be the exact reductant for the Cr(VI) reduction, quite different from the reported H2-reduction route. The enhanced catalysis originates from the electronic and geometric modification of active Pd after formation of PdCu alloy. Electron transfer from Cu to Pd enhances the electron density of Pd atoms, which favors the adsorption of the bridging formate intermediate and subsequent generation of active H atoms over PdCu/NG. The catalyst can be recycled five times without obvious loss of activity. Our work provides an example to explore the alloying effect on the catalytic behavior of PdCu alloy, which may shed light on developing other advanced nanoalloys for Cr(VI) reduction.


 Please wait while we load your content...
Please wait while we load your content...