Photodissociation dynamics of acetone studied by time-resolved ion imaging and photofragment excitation spectroscopy
Abstract
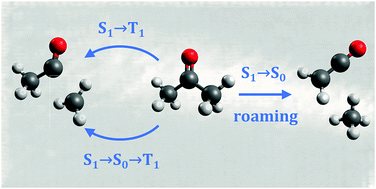
The photodissociation dynamics of acetone has been investigated using velocity-map ion imaging and photofragment excitation (PHOFEX) spectroscopy across a range of wavelengths spanning the first absorption band (236–308 nm). The radical products of the Norrish Type I dissociation, methyl and acetyl, as well as the molecular product ketene have been detected by single-photon VUV ionization at 118 nm. Ketene appears to be formed with non-negligible yield at all wavelengths, with a maximum value of Φ ≈ 0.3 at 280 nm. The modest translational energy release is inconsistent with dissociation over high barriers on the S0 surface, and ketene formation is tentatively assigned to a roaming pathway involving frustrated dissociation to the radical products. Fast-moving radical products are detected at λ ≤ 305 nm with total translational energy distributions that extend to the energetic limit, consistent with dissociation occurring near-exclusively on the T1 surface following intersystem crossing. At energies below the T1 barrier a statistical component indicative of S0 dissociation is observed, although dissociation via the S1/S0 conical intersection is absent at shorter wavelengths, in contrast to acetaldehyde. The methyl radical yield is enhanced over that of acetyl in PHOFEX spectra at λ ≤ 260 nm due to the onset of secondary dissociation of internally excited acetyl radicals. Time-resolved ion imaging experiments using picosecond duration pulses at 266 nm find an appearance time constant of τ = 1490 ± 140 ps for CH3 radicals formed on T1. The associated rate is representative of S1 → T1 intersystem crossing. At 284 nm, CH3 is formed on T1 with two distinct timescales: a fast <10 ns component is accompanied by a slower component with τ = 42 ± 7 ns. A two-step mechanism involving fast internal conversion, followed by slower intersystem crossing (S1 → S0 → T1) is proposed to explain the slow component.


 Please wait while we load your content...
Please wait while we load your content...