Modifying bis(triflimide) ionic liquids by dissolving early transition metal carbamates†
Abstract
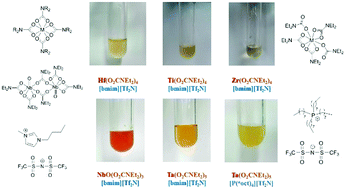
The authors report the first modification of ionic liquids with metal carbamates. A selection of homoleptic N,N-dialkylcarbamates of group 4 and 5 metals, M(O2CNR2)n, were dissolved in bis(trifluoromethylsulfonyl)imide-based ionic liquids, i.e. [bmim][Tf2N] and [P(oct)4][Tf2N], at 293 K. The resulting solutions were characterized by means of IR, UV and NMR spectroscopy, and the data were compared to those of the respective metal compounds. Notably, the dissolution process did not proceed with the release of any of the original carbamato ligands, thus preserving the intact coordination frame around the metal centre. The solvation process of Ti(O2CNiPr2)4, as a model species, in [bmim][Tf2N] was rationalized by DFT calculations. As a comparative study, solutions of NbF5 and MCl5 (M = Nb, Ta) in [bmim][Tf2N] were also investigated, revealing the possible occurrence of solvent anion coordination to the metal centres.


 Please wait while we load your content...
Please wait while we load your content...