Self-assembled uracil complexes containing tautomeric uracils: an IRMPD spectroscopic and computation study of the structures of gaseous uracilnCa2+ (n = 4, 5, or 6) complexes†
Abstract
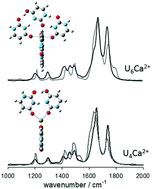
The structures of doubly-charged uracil (U) complexes with Ca2+, UnCa2+ (n = 4, 5, 6), were studied by infrared multiple photon dissociation (IRMPD) spectroscopy and computational methods. The ions were produced by electrospray ionization (ESI) and were isolated in the gas phase in a Fourier-transform ion cyclotron resonance mass spectrometer (FT-ICR-MS). The recorded IRMPD spectra in both the fingerprint and the C–H/N–H/O–H stretching regions, combined with computed vibrational spectra, reveal that the structures present in the greatest abundance consist of both canonical uracil as well as the lactam (or colloquially “enol”) tautomer of uracil. U4Ca2+ consists of two hydrogen-bonded dimers of uracil, one canonical and one tautomer, with each uracil interacting with Ca2+ through a carbonyl oxygen. The structures most consistent with the vibrational spectrum of U6Ca2+ consist of two hydrogen-bonded uracil trimers, each composed of two canonical and one enolic uracil, with each uracil also interacting with Ca2+ through carbonyl oxygen. U5Ca2+ consists of one of the aforementioned trimers and dimers, each containing one enol tautomerized uracil. The computed structures whose vibrational spectra best agree with the experimental vibrational spectra are also the lowest-energy structures for all three complexes. This study clearly shows that some uracils adopt the normally very high energy enol tautomer in the lowest energy gas phase complexes of uracil with a doubly-charged ion like Ca2+.


 Please wait while we load your content...
Please wait while we load your content...