Experimental and modelling study of the multichannel thermal dissociations of CH3F and CH2F†
Abstract
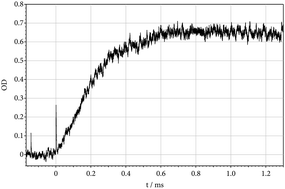
The thermal unimolecular dissociation of CH3F was studied in shock waves by monitoring the UV absorption of a dissociation product identified as CH2F. It is concluded that, under conditions applied, the formation of this species corresponds to a minor, spin-allowed, dissociation channel of about 3% yield. Near to the low-pressure limit of the reaction, on the other hand, the energetically more favourable dissociation leads to 3CH2 + HF on a dominant, spin-forbidden, pathway. By considering the multichannel character of the reaction, it is shown that, in contrast to the low-pressure range, the high-pressure range of the reaction should be dominated by CH2F formation. The channel-switching probably takes place at pressures higher than those applied in the present work. In addition to the two dissociation channels of CH3F producing 3CH2 + HF and CH2F + H, a third, spin-allowed, dissociation channel leading to 1CHF + H2 was also considered and estimated to proceed with a yield smaller than 0.5%. Besides the dissociation of CH3F, the dissociation of CH2F was studied by monitoring the UV spectrum of CH2F. Details of this spectrum were investigated. Similar to CH3F, the dissociation of CH2F can proceed on several dissociation channels, under the present conditions either to CHF + H or to CF + H2. After modelling single-channel falloff curves for all reaction pathways, coupling effects of multichannel dissociations were interpreted in the framework of multichannel unimolecular rate theory.


 Please wait while we load your content...
Please wait while we load your content...