Analysis of competitive binding of several metal cations by graphene oxide reveals the quantity and spatial distribution of carboxyl groups on its surface†
Abstract
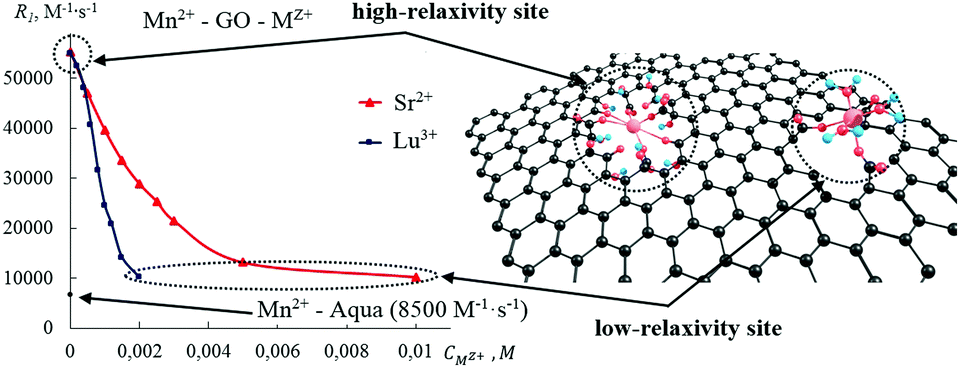
The sorption capacity of graphene oxide (GO) toward different metal cations has been the subject of several recent studies. However, the reported quantitative data are controversial, and the mechanism of chemical bonding between GO and metal cations is poorly understood. Clarifying these questions can eventually help to reveal the fine chemical structure of GO that remains ambiguous. In this work, we study the binding of Gd3+ and Mn2+ by GO in the presence of several competing metal cations by the 1H NMR relaxation method. As a general trend, the efficiency of the metal cations to bind to GO increases with ionic charge, and depends on their ability to form coordinate-covalent bonds with GO oxygen groups. The efficiency of the competing metal cations to “replace” Gd3+ and Mn2+ increases in the order Na+ < Cs+ < Ca2+ < Sr2+ < Ga3+ < Lu3+. GO contains two different types of binding sites, bonding to which results in either high or low NMR relaxivity of the resulting Gd3+–GO and Mn2+–GO solutions. Gd3+ and Mn2+, being replaced from the high-relaxivity sites by the large excess of competing cations, are not released into the bulk solution, but only migrate to the low-relaxivity sites, remaining covalently bonded to GO. The absolute majority of the existing carboxyl groups in GO are located at tiny few-carbon-atom-vacancy defects on the major planes. The density of these vacancy defects is estimated as one per every 200 carbon atoms.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...