Effects of salinity on the adsorption of nucleotides onto phyllosilicates†
Abstract
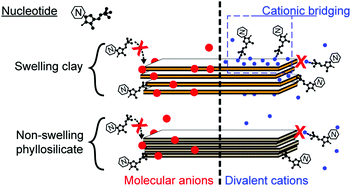
In the context of the origin of life, phyllosilicate surfaces might favor the adsorption, concentration and reactivity of otherwise diluted prebiotic molecules. The primitive oceanic seafloor was certainly rich in Fe–Mg-rich phyllosilicates. The salinity of the primitive seawater remains largely unknown. Values ranging from 1 to 15 times modern salinity have been proposed and the salt composition of the primitive ocean also remains elusive although it may have played a role in the interactions between nucleotides and mineral surfaces. Therefore we studied the adsorption of 5′-monophosphate deoxyguanosine (dGMP) as a model nucleotide onto a Fe-rich swelling clay, i.e. nontronite, and an Al-rich phyllosilicate, i.e. pyrophyllite, for comparison. Experiments were carried out at atmospheric pressure, 25 °C and natural pH, with a series of salts NaCl, MgCl2, CaCl2, MgSO4, NaH2PO4 and LaCl3 in order to evaluate the effect of cations and anions on dGMP adsorption. The present study shows that nucleotides are adsorbed on both phyllosilicates via a ligand exchange mechanism. The phosphate group of the nucleotide is adsorbed on the lateral metal hydroxyls of the broken edges of phyllosilicates. The presence of divalent cations or molecular anions, such as phosphate or sulfate, tends to inhibit this interaction on mineral surfaces. However, in the presence of divalent cations, cationic bridging on the basal surfaces of the swelling clay also occurs and could induce a higher retention capacity of the swelling clays compared to non-swelling phyllosilicates in primitive and modern natural environments.


 Please wait while we load your content...
Please wait while we load your content...