Aqueous TMAO solutions as seen by theoretical THz spectroscopy: hydrophilic versus hydrophobic water
Abstract
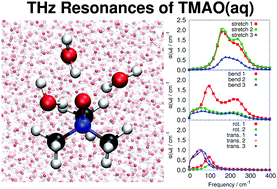
Solvation of trimethylamine-N-oxide (TMAO) by water is of great fundamental interest because this small molecule has both strongly hydrophilic and large hydrophobic groups at its opposite ends and, furthermore, stabilizes proteins against temperature and pressure denaturation. Since hydrophilic and hydrophobic groups affect the structural dynamics of the respective solvation water molecules in vastly different ways, we dissect their distinct influences on the THz spectrum of TMAO(aq) by using ab initio molecular dynamics simulations. In particular, we demonstrate that exclusively electronic polarization and charge transfer effects, being absent in the usual fixed-charge biomolecular force fields, are responsible for the significant enhancement of the effective molecular dipole moment of hydrophilic solvation water. This, in turn, leads to pronounced solute–solvent couplings and thus to specific THz modes that involve well-defined H-bond bending and stretching motion being characteristic to hydrophilic solvation. The THz response of individual H-bonded pairs of water molecules involving hydrophobic solvation water, in stark contrast, is nearly indistinguishable from such pairs in bulk water. Transcending the specific case, THz spectroscopy is suggested to be an ideal experimental approach to unravel the controversial piezolytic properties of TMAO including its counteracting effect on pressure-induced denaturation of proteins.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...