Theoretical investigation of selective catalytic reduction of NO on MIL-100-Fe
Abstract
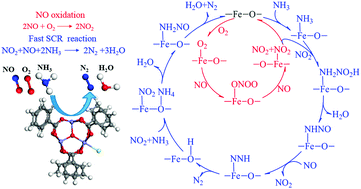
MIL-100-Fe, a three-dimensional porous metal organic framework material, shows good activity for the selective catalytic reduction of NO by ammonia. Understanding reaction mechanisms at the molecular level contributes to the design and development of highly active catalysts. Herein, density functional theory calculations with the consideration of van der Waals interactions were performed to investigate the SCR mechanism on MIL-100-Fe. Lewis acid sites and Brønsted acid sites both exist on MIL-100-Fe, which are active for the SCR reaction. MIL-100-Fe exhibits the features of single-site catalysts, thus we calculated the NH3-SCR reaction process following the Eley–Rideal mechanism. The proposed NH3-SCR mechanism can be subdivided into two parts: (1) NO oxidation and (2) fast SCR reaction. Our results show that Lewis acid sites play an essential role in activating the reactants. The NO molecule is readily oxidized to NO2 species on the Fe Lewis acid sites, the adsorbed NH3 species react with gaseous NO2, and the formation of intermediate NH2NO is the rate-determining step. This study offers new and important insights into the mechanistic understanding of the NH3-SCR reaction on the MIL-100-Fe catalyst.


 Please wait while we load your content...
Please wait while we load your content...