Cluster assemblies as superatomic solids: a first principles study of bonding & electronic structure†
Abstract
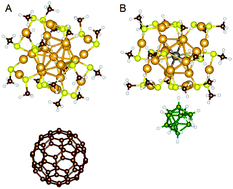
The synthesis of cluster based materials poses an exciting challenge for experimental chemistry. The main advantage of these materials compared to conventional bulk compounds is the simple tunability of the chemical and physical characteristics of individual clusters. As a consequence, cluster assemblies can theoretically be used for the creation of designer materials exhibiting specifically desired properties. Since superatoms reveal a large intrinsic thermodynamic stability and often very interesting tunable electronic characteristics, they seem to be an excellent choice as building blocks for the bulk. Here, we present a detailed first principles analysis of carefully chosen superatomic cluster binary and bulk assemblies, in order to determine which forces control the attractive interaction in superatomic solids, and how the individual cluster properties affect these assemblies. This study uses the highly tunable and stable Au13(RS(AuSR)2)6 cluster with a variety of dopants as a model system, while the principles are likely transferable to other ligand protected systems with a straightforward superatomic electron count, such as aluminum or sodium clusters. Three different superatomic materials based on doped gold clusters, boranes and C60s are constructed and evaluated. Beyond the verification that superatoms can be used to create materials that reveal emergent atom-based solid like properties, various factors influencing superatomic materials, such as the EA, IP and relative sizes of the clusters, have been identified and critically evaluated.


 Please wait while we load your content...
Please wait while we load your content...