Kinetics of the simplest Criegee intermediate reaction with ozone studied using a mid-infrared quantum cascade laser spectrometer†
Abstract
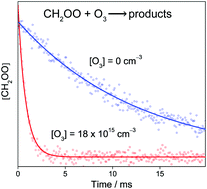
The kinetics of the reaction of CH2OO with ozone has been studied by monitoring CH2OO using time-resolved infrared (IR) absorption spectroscopy, which utilized the fast chirped IR pulse train from a quantum cascade laser [J. Chem. Phys., 2017, 146, 244302]. CH2OO was prepared by photolyzing a gas mixture of CH2I2/O2/O3 at 352 nm; the photolysis wavelength was chosen to minimize the photodissociation of O3. The measured rate coefficient at 298 K and 30 Torr is (6.7 ± 0.5) × 10−14 cm3 s−1, independent of pressure from 30 to 100 Torr. The result indicates that previous ab initio calculations either underestimated or overestimated this reaction rate by one order of magnitude or more. The result also implies that, the reaction of the Criegee intermediate with ozone may play a role in laboratory studies of ozonolysis of alkenes. However, this reaction would not compete with other CH2OO sinks in the atmosphere.


 Please wait while we load your content...
Please wait while we load your content...