L-edge sum rule analysis on 3d transition metal sites: from d10 to d0 and towards application to extremely dilute metallo-enzymes†
Abstract
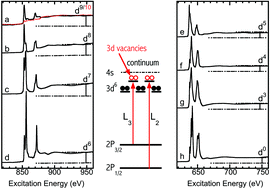
According to L-edge sum rules, the number of 3d vacancies at a transition metal site is directly proportional to the integrated intensity of the L-edge X-ray absorption spectrum (XAS) for the corresponding metal complex. In this study, the numbers of 3d holes are characterized quantitatively or semi-quantitatively for a series of manganese (Mn) and nickel (Ni) complexes, including the electron configurations 3d10 → 3d0. In addition, extremely dilute (<0.1% wt/wt) Ni enzymes were examined by two different approaches: (1) by using a high resolution superconducting tunnel junction X-ray detector to obtain XAS spectra with a very high signal-to-noise ratio, especially in the non-variant edge jump region; and (2) by adding an inert tracer to the sample that provides a prominent spectral feature to replace the weak edge jump for intensity normalization. In this publication, we present for the first time: (1) L-edge sum rule analysis for a series of Mn and Ni complexes that include electron configurations from an open shell 3d0 to a closed shell 3d10; (2) a systematic analysis on the uncertainties, especially on that from the edge jump, which was missing in all previous reports; (3) a clearly-resolved edge jump between pre-L3 and post-L2 regions from an extremely dilute sample; (4) an evaluation of an alternative normalization standard for L-edge sum rule analysis. XAS from two copper (Cu) proteins measured using a conventional semiconductor X-ray detector are also repeated as bridges between Ni complexes and dilute Ni enzymes. The differences between measuring 1% Cu enzymes and measuring <0.1% Ni enzymes are compared and discussed. This study extends L-edge sum rule analysis to virtually any 3d metal complex and any dilute biological samples that contain 3d metals.


 Please wait while we load your content...
Please wait while we load your content...