Solvent and concentration effects on highly defined, colloid-like ionic clusters in solution†
Abstract
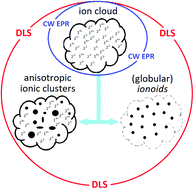
We characterize the process of ionic self-assembly involving a macrocyclic tetraimidazolium molecular box and small dianionic salts into highly defined, colloid-like ionic clusters called ionoids. Using dynamic light scattering (DLS) and continuous-wave electron paramagnetic resonance (CW EPR) spectroscopy, we determine the influence of solvent composition and bulk concentration on the size, shape and durability of the ionoids. Minor aberrations from the established optimum solvent mixture DMSO : glycerol : water 50 : 43 : 7 (v/v/v) in volume stoichiometry still lead to formation of ionoids, but on longer timescales they evolve into separated smaller and bigger entities. The same effect occurs when decreasing the bulk concentration from approximately 1 mM : 3 mM to 10-fold and higher dilutions. This transition at the 0.1 mM : 0.3 mM ratio represents the ‘critical ionoid concentration’ for the investigated system. We can thus define the solvent and concentration range, in which these soft, yet durable and long lived colloid-like ionic clusters form.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...