Enhanced thermoelectric performance of Cu3SbS4 flower-like hierarchical architectures composed of Cl doped nanoflakes via an in situ generated CuS template†
Abstract
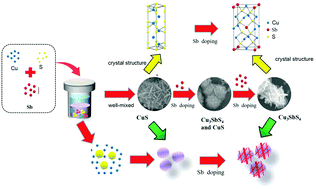
In this work, Cu3SbS4 hierarchical flower-like microspheres composed of chlorine (Cl−)-doped Cu3SbS4 nanoflakes are realized via a one pot solvothermal ion exchange reaction. The kinetic factors including the duration time, the ratio of source materials, and the KOH concentration, are systematically investigated. Using a suite of analytical techniques, including SEM, XRD and FTIR, the mechanism of the two stage in situ chemical transformation of CuS flower-like microspheres consisting of nanoflake intermediates to the target product Cu3SbS4 is elucidated. The difference in solubility between reactants and products (Ksp(CuS) > Ksp(CuSbSx)) determines that the ion-exchange reaction from transition binary to ternary metal chalcogenides is favorable under the impetus of a thermodynamic driving force. In addition, the optical and enhanced thermoelectric transport properties are investigated. The results revealed that Cl-doped Cu3SbS4 exhibited an improved power factor, which was 8 times higher than that of undoped Cu3SbS4 at 500 K. The current study not only provides a facile and economical way to synthesize high-quality Cl-doped Cu–Sb–S three dimensional (3D) hierarchical nanostructures, but also opens up a new route for preparation of other I–V–VI multicomponent chalcogenide NCs, such as Cu–Bi–S and Cu–Pb–S systems, which would be difficult to obtain otherwise.


 Please wait while we load your content...
Please wait while we load your content...