Photochemical transformation of lipoic acid-based ligands: probing the effects of solvent, ligand structure, oxygen and pH†
Abstract
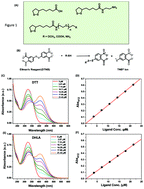
We have combined optical absorption with the Ellman's test to identify the parameters that affect the transformation of the 5-membered dithiolanes to thiols in lipoic acid (LA) and its derivatives during UV-irradiation. We found that the nature and polarity of the solvent, the structure of the ligands, acidity of the medium and oxygen can drastically affect the amount of photogenerated thiols. These findings are highly relevant to the understanding of the photochemical transformation of this biologically relevant compound, and would benefit the increasing use of LA-based ligands for the surface functionalization of various nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...