Controllable dissociation of H2O on a CeO2(111) surface†
Abstract
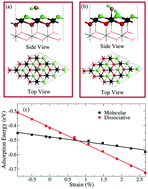
Splitting water on metal oxide surfaces plays a key role in surface catalytic reactions. Continuously tuning the molecular/dissociative states of H2O can be expected to achieve controllable catalysis. In this work, we propose a promising method to split water on a CeO2(111) surface and uncover the dissociation mechanism. According to first-principles calculations, we demonstrate that tensile strain facilitates water dissociation on the CeO2(111) surface due to the enhancement of hybridization between the 4f states of surface Ce and the 2p states of the dissociated H2O. More importantly, strain-induced water dissociation could also apply to other metal oxide surfaces, such as PaO2(111), ThO2(111), and CaO(100). It is proposed that different behaviors of the oxygen 2p band center shift for molecular and dissociative H2O are responsible for tensile strain-induced water dissociation on metal oxide surfaces. The results indicate that Ag(111) or Au(111) can serve as substrates to realize water dissociation on ultra-thin CeO2(111) films. Our studies provide an effective approach for tuning the surface reactions of metal oxide surfaces by modulating lattice parameters.


 Please wait while we load your content...
Please wait while we load your content...