Dissociative ionisation of adamantane: a combined theoretical and experimental study†
Abstract
Diamond nanoparticles, or nanodiamonds, are intriguing carbon-based materials which, maybe surprisingly, are the most abundant constituent of presolar grains. While the spectroscopic properties of even quite large diamondoids have already been explored, little is known about their unimolecular fragmentation processes. In this paper we characterise the dissociative ionisation of adamantane (C10H16) – the smallest member of the diamondoid family – utilising imaging Photoelectron Photoion Coincidence (iPEPICO) spectroscopy and Density Functional Theory (DFT) calculations. We have found adamantane to dissociatively photoionise via several parallel channels of which H, C3H7 and C4H8 losses are the most important ones. Calculations confirm the existence of a rate-limiting transition state for the multiple C-loss channels, which is located at 10.55 eV with respect to neutral adamantane. In addition, we found dissociation channels leading to small cationic hydrocarbons, which may be relevant in the interstellar medium.
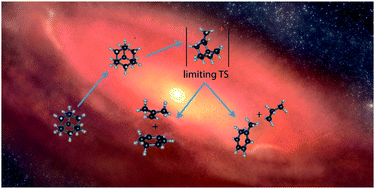
- This article is part of the themed collections: 2018 PCCP HOT Articles and Theory, experiment, and simulations in laboratory astrochemistry


 Please wait while we load your content...
Please wait while we load your content...