Binding energies of hydrated cobalt(ii) by collision-induced dissociation and theoretical studies: evidence for a new critical size†
Abstract
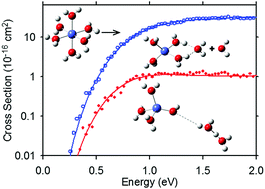
The experimental sequential bond energies for loss of water from Co2+(H2O)x complexes, x = 5–11, are determined by threshold collision-induced dissociation (TCID) using a guided ion beam tandem mass spectrometer with a thermal electrospray ionization source. Kinetic energy dependent TCID cross sections are analyzed to yield 0 K thresholds for sequential loss of neutral water molecules. The thresholds are converted from 0 to 298 K values to give hydration enthalpies and free energies. Theoretical geometry optimizations and single point energy calculations at several levels of theory are performed for the reactant and product ion complexes. Theoretical bond energies for ground structures are used for direct comparison with experimental values to obtain structural information on these complexes. In addition, the dissociative charge separation process, Co2+(H2O)x → CoOH+(H2O)m + H+(H2O)x−m−1, is observed at x = 4, 6, and 7 in competition with primary water loss products. Energies for the charge separation rate-limiting transition states are calculated and compared to experimental threshold measurements. Results suggest that the critical size for which charge separation is energetically favored over water loss is xcrit = 6, in contrast to lower values in previous literature reports.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...