Structure analysis of collagen fibril at atomic-level resolution and its implications for intra-fibrillar transport in bone biomineralization
Abstract
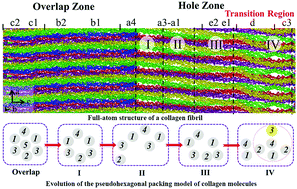
Bone is a hierarchical biocomposite material in which a collagen fibril matrix self-assembled in a three-dimensional (3-D) pseudohexagonal array controls many important processes in mineralization such as providing the pathways by which calcium and phosphate species are delivered and a template for the earliest nucleation sites, determining the spatial distribution of the mineral and the topology for binding of associated non-collagenous proteins. However, the structural characteristics of collagen molecules in the fibril remain unclear at the atomic level. Here we performed the first large-scale molecular dynamics simulations to provide a comprehensive all-atom structural analysis of the entire fibril of Type I collagen including intra-fibrillar water distribution. We found that the ideal fibril structure is preserved in specific sites where the earliest nucleation occurs, but is severely distorted in areas that mineralize later. In detail, the ideal pseudohexagonal structure is well-preserved in the overlap zone (c1, c2 and b bands), in the a bands of the hole zone but is severely distorted at the hole/overlap transition (d and c3 bands). As a result, the expected uniform “channel,” formed by connecting holes in adjacent unit cells along the b-axis, and having dimensions of 1.5 nm height along the a-axis and width of 40 nm along the c-axis is not formed. The expected uniform channel of 1.5 nm height is preserved only in the a bands in a narrow sub-channel region only 5.8 nm wide. At the hole/overlap transition, an irregular, tortuous sub-channel of widely varying dimensions (∼1.8–4.0 nm height × ∼3.0 nm width) is formed. The well-defined sub-channel in the a bands along with their preferred orientation of charged amino acid residues could facilitate faster molecular diffusion than the tortuous sub-channels and ionic interactions, thus providing the first nucleation sites. Intra-fibrillar water occupies nano-spaces and shows low density (∼0.7 g cm−3), which should promote dehydration of ion species. These results provide the first atomic-level understanding of the structure of the collagen fibril and the properties of the aqueous compartments within the fibril, which offer a physical, chemical and steric explanation for calcium phosphate infiltration paths and for the initiation of mineralization at the a band collagen fibril. The mechanism revealed here for the observed specificity of collagen biomineralization in bone formation ultimately contributes to the biochemical and biomechanical functions of the skeleton.


 Please wait while we load your content...
Please wait while we load your content...