Non-equilibrium fractal growth of MoS2 for electrocatalytic hydrogen evolution†
Abstract
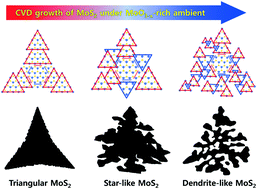
Non-equilibrium fractal growth of MoS2 was induced by establishing an extremely Mo rich chemical vapor deposition (CVD) environment using a rapid heating rate in a confined reaction space. In addition, a detailed shape evolution mechanism was suggested based on the results of thermodynamics and kinetics studies. The extremely Mo rich CVD condition allowed various MoS2 flakes to be synthesized with triangular, star-like, and dendrite-like shapes in series, owing to the thermodynamic preference to increase the number of growth directions. Moreover, numerous small triangular crystals led to the formation of MoS2 flakes with jagged edges, probably as a result of the aggressive nucleation triggered by a fast and abundant supply of MoO3−x vapors. Lastly, using the MoS2 flakes with various fractal shapes, a systematic improvement of the hydrogen evolution reaction performance was demonstrated in accordance with the increase in the surrounding edge length.


 Please wait while we load your content...
Please wait while we load your content...