Low dimensional and frustrated antiferromagnetic interactions in transition metal chloride complexes with simple amine ligands†
Abstract
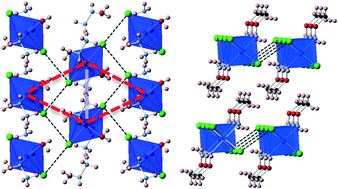
This study reports the facile synthesis, crystal structures and magnetic properties of five new Mn, Co and Cu complexes with chloride and simple amine ligands. The four hydrazinium complexes are discrete in nature while the O-methylhydroxylamine phase contains edge-sharing chains bridged by chloride ligands. Investigation of the magnetic properties of these materials reveals that two of these materials, Co(NH3NH2)2(H2O)2Cl4 and Cu(NH2OCH3)2Cl2, exhibit interesting antiferromagnetic properties arising from their low dimensional structures. Co(NH3NH2)2(H2O)2Cl4 appears to exhibit significant 2D magnetic frustration while the magnetic susceptibilities of Cu(NH2OCH3)2Cl2 are well fitted by a one-dimensional chain model. The relationships between the strength of the magnetic coupling observed in these materials and their likely exchange pathways are also discussed.


 Please wait while we load your content...
Please wait while we load your content...