Alkali metal salts of 3,6-dinitramino-1,2,4,5-tetrazine: promising nitrogen-rich energetic materials†
Abstract
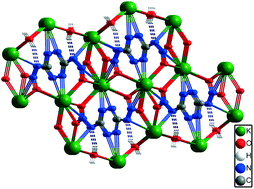
The alkali metal salts of 3,6-dinitramino-1,2,4,5-tetrazine (DNAT, 1), Li2(H2O)4(DNAT) (2), Na2(H2O)3(DNAT) (3), K2(H2O)(DNAT) (4), Rb2(DNAT) (5) and Cs2(DNAT) (6) were synthesized and characterized by elemental analysis, IR spectroscopy, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and single-crystal X-ray diffraction. All of these alkali metal salts possess higher thermal stability (from 240.9 °C to 260.0 °C) than that of DNAT substrate (108.3 °C) due to their numerous coordination bonds and the three-dimensional network structures of the complexes. The number of coordination water molecules, the atomic radius of metal and the different packing forms of the crystal structure contribute to the different thermal stabilities of the alkali metal salts. The experimental (constant-volume) energy of combustion values range from 1331 to 1531 kJ mol−1. The compounds 1, 4, 5, and 6 are sensitive to impact and friction.


 Please wait while we load your content...
Please wait while we load your content...