A novel homochiral metal–organic framework with an expanded open cage based on (R)-3,3′-bis(6-carboxy-2-naphthyl)-2,2′-dihydroxy-1,1′-binaphthyl: synthesis, X-ray structure and efficient HPLC enantiomer separation†
Abstract
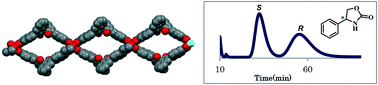
A new homochiral metal–organic framework (MOF) with an expanded open cage based on the (R)-3,3′-bis(6-carboxy-2-naphthyl)-2,2′-dihydroxy-1,1′-binaphthyl ligand was synthesized and utilized as a novel chiral stationary phase for high-performance liquid chromatography. Twelve racemates including sec-alcohols, sulfoxides, epoxides, lactone, 1,3-dioxolan-2-one, and oxazolidinone were used as analytes for evaluating the separation properties of the chiral-MOF-packed column. Experimentally, the homochiral MOF offered good molecular recognition ability, which suggests good prospects for the application of chiral MOFs as stationary phases for enantioseparation.


 Please wait while we load your content...
Please wait while we load your content...