Computational and analytical approaches for investigating hydrates: the neat and hydrated solid-state forms of 3-(3-methylimidazolium-1-yl)propanoate†
Abstract
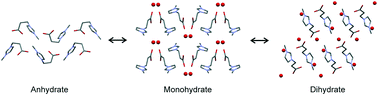
3-(3-Methylimidazolium-1-yl)propanoate (OOCEMIM), the related zwitterion of the parent ionic liquid [HOOCEMIM] [Cl], crystallises in two hydrated and one neat (AH) solid-state forms. All three forms can be produced directly in solution crystallisation experiments and their single crystal structures as well as the temperature- and moisture dependent interconversion pathways between the forms were unravelled. The OOCEMIM molecule adopts an elongated high energy conformation in both the mono- (Hy1) and dihydrates (Hy2), with water⋯OOCEMIM interactions contributing significantly to the lattice energy. A substantial conformational rearrangement of OOCEMIM is observed upon dehydration, resulting in a bent low energy conformation in the anhydrous form. Each of the three OOCEMIM solid-state forms shows a limited existence range. The anhydrous form AH can only be preserved at very dry atmospheric conditions (at 25 °C). The exposure of AH to elevated water vapour conditions results in a fast transformation to Hy1, which then transforms to Hy2 at relative humidities (RH) above 18%. Increasing the RH above 40% leads to deliquescence of the zwitterion. Hy1 and Hy2 melt at 90 °C and 61 °C (peritectic dissociation temperature), respectively, whereas AH melts and decomposes at 172 °C. The measured transformation enthalpies between the three solid-state forms, derived from differential scanning and isothermal calorimetry experiments, are in excellent agreement with the 0 K PBE-TS and PBE-D2 lattice energy differences. Knowledge about the interconversion pathways and stability ranges of the OOCEMIM solid-state forms is essential for handling the compound.
- This article is part of the themed collection: Editor’s Collection: Computer aided solid form design


 Please wait while we load your content...
Please wait while we load your content...