Prediction of piezoelectric properties by first principles calculations and hydrothermal crystal growth of Si1−xSnxO2 α-quartz phase†
Abstract
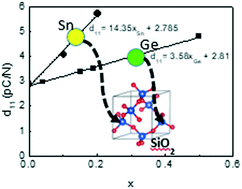
First principles calculations of the α-quartz phase of Si1−xSnxO2 predict great benefits of the substitution of Si by Sn with the d11 piezoelectric constant (5.72 pC N−1) for Si0.8Sn0.2O2, which is predicted to be twice that of quartz (2.85 pC N−1). Furthermore, the value of the d11 piezoelectric constant increases four times in the case of substitution with tin in comparison with that for germanium. The first Sn-substituted α-quartz single crystals have been grown under hydrothermal conditions, but the degree of substitution is still very low (xmax = 0.001). The analyses of additional compounds recovered after growth runs indicate that Sn crystallizes in a six-fold coordination with silicon by forming a mixed Si/Sn oxide in the autoclave, which may explain the low degree of substitution in the α-quartz single crystals. A new compound with the formula Na2Si4SnO9(OH)4 is identified, and the structure is determined. This paper predicts high performance of the Sn-substituted single quartz crystals. Crystal growth experiments show that Sn substitution is possible in the structure of quartz-type by using a crystal growth process favoring the formation of four-fold coordinated Sn species [SnO4] in the solution for obtaining a high Sn ratio in the quartz-crystals.


 Please wait while we load your content...
Please wait while we load your content...