Morphological evolution of Co phosphate and its electrochemical and photocatalytic performance†
Abstract
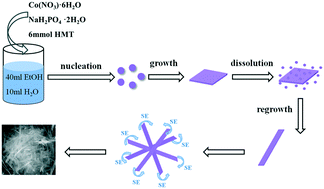
Cobalt phosphates with different compositions and morphologies were prepared via a one-step hydrothermal method. Interestingly, a novel morphology resembling a self-curled piece of cotton consisting of nanobelts was created under the optimized preparation conditions. The amount of hexamethylenetetramine (HMT) and the ratio of mixed solvent greatly affected the morphology, size and phase components. The morphology changed from flower-like to cotton-like by changing the amount of HMT. As the proportion of ethanol in the mixed solvent increased, the morphology changed from flower-like nanosheet to cotton-cluster nanobelt, the aspect ratio gradually increased, and the phase composition changed from cobalt phosphate octahydrate to cobalt phosphate tetrahydrate. The electrochemical properties and photocatalytic performances of the samples were also investigated. Co-Based phosphate exhibited good capacitance behavior and cycle stability and enhanced the photocatalytic degradation of rhodamine B in the visible-light range.


 Please wait while we load your content...
Please wait while we load your content...