Halide ion recognition via chalcogen bonding in the solid state and in solution. Directionality and linearity†
Abstract
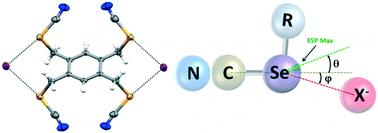
Group 16 chalcogen atoms may exhibit Lewis acidic σ-holes which are able to form attractive supramolecular interactions with Lewis bases via chalcogen bonds (ChB). Interest in ChB is increasing rapidly; however, the potential for anion binding has not been fully explored. Herein we report on the application of chemically robust and stable benzylic selenocynates (1 and 2) for halide ion recognition in the solid state and in solution. Single crystal X-ray structural analysis of various cocrystals reveals structurally important Se⋯X− (X = Cl, Br, I) chalcogen bonds. Changes in the 13C and 77Se chemical shifts via NMR spectroscopy show how halide ion recognition influences the local electronic environment of the selenium atoms in solution. Deviations of the interaction from the extension of the C–Se covalent bond are quantified and assessed with the aid of computational chemistry.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...