Zwitterion formation and subsequent carboxylate–pyridinium NH synthon generation through isomerization of 2-anilinonicotinic acid†
Abstract
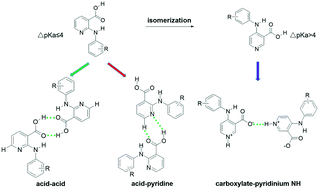
Through structural modification of 2-anilinonicotinic acid by isomerization, the ΔpKa between the carboxylic acid and pyridinium NH increased dramatically over the threshold to form zwitterions in the newly designed 4-anilinonicotinic acids. This in turn led to the formation of a hydrogen bond between carboxylate and pyridinium NH, and the absence of two commonly observed synthons, i.e., the acid–acid homosynthon and acid–pyridine heterosynthon. The new synthon has a stronger hydrogen bond than the acid–acid homosynthon and the acid–pyridine heterosynthon, as suggested by theoretical calculations. This, together with the much larger ΔpKa, explains its formation.


 Please wait while we load your content...
Please wait while we load your content...