Halogen bonding at the wet interfaces of an amyloid peptide structure†
Abstract
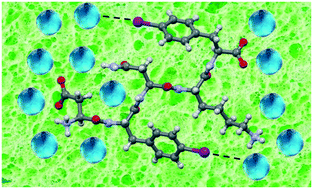
Amyloid peptide hydrogels are a class of materials of great interest due to their structural simplicity, good performances and easy tuning of their properties by chemical modification. Among the possible modifications, halogenation has not yet been exploited extensively. Here, we report the single-crystal X-ray structure of two dihalogenated derivatives of the amyloidogenic sequence DFNKF. The obtained results show how halogenation is a promising tool to stabilize – through halogen bonds – the wet interface of amyloid structures, to determine an increase in the water uptake, hence the hydrogelation properties of the peptide sequence.
- This article is part of the themed collections: Halogen Bonding in Crystal Engineering Editor’s collection and CrystEngComm 20th volume collection


 Please wait while we load your content...
Please wait while we load your content...