Joining of trinuclear (CuL)2M (M = MnII and CdII) nodes by 1,3- and 1,4-benzenedicarboxylate linkers: positional isomeric effect on co-crystallization†
Abstract
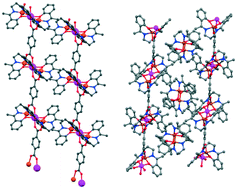
Four new one-dimensional coordination polymers {[(CuL)2Cd(p-BDC)]·H2O}n (1), {[(CuL)2Mn(p-BDC)]·H2O}n (2), {[(CuL)2Cd(m-BDC)][(CuL)2]}n (3), and {[(CuL)2Mn(m-BDC)][(CuL)2]}n (4), where H2L = N,N′-bis(α-methylsalicylidene)-1,3-propanediamine, have been synthesized using bimetallic trinuclear nodes, [(CuL)2M] (where M is MnII/CdII and p-BDC and m-BDC are 1,4- and 1,3-benzenedicarboxylate, respectively). Crystal structures show that all four complexes form 1D chains in which the heterometallic linear trinuclear nodes are connected by syn–syn bridging dicarboxylate linkers. However, in 1 and 2 the nodes which are connected by linear p-BDC are parallel to each other whereas in 3 and 4 the nodes are inclined towards each other to be connected by the angular m-BDC linker. Moreover, complexes 3 and 4 are co-crystallized with dimeric CuII units which are accommodated in the cavities between polymeric chains. This difference in the solid-state crystal packing is explained theoretically by DFT calculations of complexes 1 and 3 considering the positional isomeric effect of dicarboxylate linkers. Variable temperature magnetic susceptibility measurements of complexes 2 and 4 indicate antiferromagnetic interactions between the CuII and MnII centers with J = −13.08 and −12.11 cm−1 for 2 and 4, respectively.


 Please wait while we load your content...
Please wait while we load your content...