Halogen-bonded cocrystals of N-salicylidene Schiff bases and iodoperfluorinated benzenes: hydroxyl oxygen as a halogen bond acceptor†
Abstract
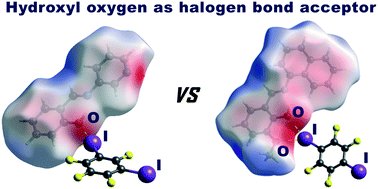
To explore the halogen bonding proclivity of the o-hydroxyimine hydroxyl group, we have prepared two imines as halogen bond acceptors: salicylideneaniline (I) and a 3-pyridyl analogue (II) derived from the condensation of salicylaldehyde and 3-aminopyridine. These two Schiff bases were selected as the two simplest representatives of o-hydroxyimines, where one (II) possesses a potentially competing halogen bond acceptor (pyridine nitrogen) and the other does not. For cocrystal screening, as halogen bond donors, we used perfluorinated iodobenzenes: 1,2-, 1,3-, 1,4-diiodotetrafluoro-benzene (12tfib, 13tfib, and 14tfib) and 1,3,5-triiodotrifluoro-benzene (135tfib). The hydroxyl group has been found to act as a halogen bond acceptor in three out of five crystal structures determined in this study: (II)(13tfib), (II)(135tfib) and (II)2(135tfib). In all three cases, a pyridine nitrogen is also employed in halogen bonding. Our attempts at preparing cocrystals of I were generally unsuccessful and the only cocrystal of I, which has been obtained, with 14tfib, does not exhibit a halogen bond involving a hydroxyl oxygen. These results suggest that the halogen bond motif with an isolated hydroxyl group as the acceptor seems to be less favourable than the previously studied bifurcated halogen bonding motif with the ortho-methoxy–hydroxyl group as the acceptor.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...