Role of hydrogen bonding in cocrystals and coamorphous solids: indapamide as a case study†
Abstract
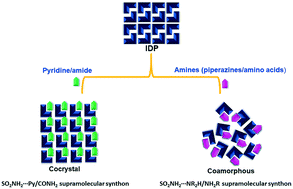
The stronger sulfonamide–pyridine (SO2NH2⋯N-Py) and sulfonamide–carboxamide (SO2NH2⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH) hydrogen bonds direct the formation of cocrystals, while the weaker sulfonamide–amine (SO2NH2⋯N–H) hydrogen bond results in coamorphous products. IDP–PIP and IDP–ARG coamorphous solids exhibit remarkable stability under accelerated conditions.
C–NH) hydrogen bonds direct the formation of cocrystals, while the weaker sulfonamide–amine (SO2NH2⋯N–H) hydrogen bond results in coamorphous products. IDP–PIP and IDP–ARG coamorphous solids exhibit remarkable stability under accelerated conditions.
- This article is part of the themed collections: Introducing the CrystEngComm Advisory Board and their research and The Solid State of Pharmaceuticals


 Please wait while we load your content...
Please wait while we load your content...