Polymorphism and luminescence properties of heteropolynuclear metal–organic frameworks containing oxydiacetate as linker†
Abstract
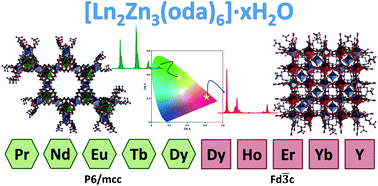
Using the flexible ligand oxydiacetic acid (H2oda), ten new heteropolynuclear compounds with general formula [Ln2Zn3(oda)6]·xH2O (Ln = lanthanide ion, oda = oxydiacetate) were synthesized and structurally characterized. They were prepared by the direct reaction of oda in aqueous solution with stoichiometric amounts of Zn and Ln salts, followed by slow organic solvent diffusion. Two different structures were obtained: the larger lanthanides Pr, Nd, Eu and Tb gave compounds belonging to the hexagonal system, space group P6/mcc; whereas, the smaller ones, Ho, Er, Yb and Y formed cubic systems, space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c. In the case of Dy, the two polymorphs were obtained by slight variations in the synthetic conditions. The phase change observed is discussed in comparison with previously reported [Ln2M3(oda)6]·xH2O compounds (M = bivalent metal ion). Luminescence studies were also carried out. Compounds containing Eu and Tb showed the most intense luminescence, also bearing relatively long lifetimes for the excited state deactivation. The luminescence profiles of both Dy systems are independent of the hexagonal or cubic arrangements, due to the similar environment of the Ln having the same point symmetry.
c. In the case of Dy, the two polymorphs were obtained by slight variations in the synthetic conditions. The phase change observed is discussed in comparison with previously reported [Ln2M3(oda)6]·xH2O compounds (M = bivalent metal ion). Luminescence studies were also carried out. Compounds containing Eu and Tb showed the most intense luminescence, also bearing relatively long lifetimes for the excited state deactivation. The luminescence profiles of both Dy systems are independent of the hexagonal or cubic arrangements, due to the similar environment of the Ln having the same point symmetry.


 Please wait while we load your content...
Please wait while we load your content...