Halogen⋯halogen interactions in the self-assembly of one-dimensional 2,2′-bipyrimidine-based CuIIReIV systems†
Abstract
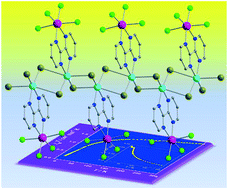
Two one-dimensional CuIIReIV coordination polymers of the general formula {[ReIVCl4(μ-bpym)CuIIX2]·solvent}n [where bpym = 2,2′-bipyrimidine, X = Cl (1) and Br (2), and solvent = H2O (1) and CHCl3 (2)] have been prepared and characterised structurally and magnetically. Both compounds crystallise in the monoclinic system with space groups P21/c (1) and P21/n (2). Each CuII ion is bonded to two cis nitrogen atoms from the bpym ligand and to four halogen atoms. Two of these halogen atoms are placed in the equatorial plane and the other two are filling the axial positions of the CuII ion, thus generating a distorted octahedral environment for this metal ion in 1 and 2. In both compounds, the ReIV ion is six-coordinate and bonded to four chloride ions and two nitrogen atoms of the bpym ligand giving rise to a distorted octahedral geometry. In the crystal packing of both compounds, hydrogen bonds (1), short intermolecular ReIV–Cl⋯Cl–ReIV contacts (1 and 2), and X⋯π type interactions (1 and 2), the intermolecular interactions causing the self-assembly of these novel one-dimensional 2,2′-bipyrimidine-based CuIIReIV systems, are present. Variable-temperature magnetic measurements performed on microcrystalline samples of 1 and 2 reveal an antiferromagnetic behaviour for both compounds. Several magnetic interactions take place in 1 and 2, both intermolecular (ReIV–ReIV) and intramolecular (ReIV–CuII and CuII–CuII) magnetic exchanges, which account for the maxima observed in their respective magnetic susceptibility versus temperature curves at ca. 6.0 (1) and 18.0 K (2).


 Please wait while we load your content...
Please wait while we load your content...