Coordination preference of hexa(2-pyridyl)benzene with copper(ii) directed by hydrogen bonding†
Abstract
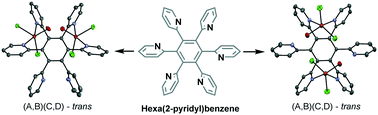
A study of the coordination chemistry of the hexa(2-pyridyl)benzene (2-HPB) ligand with copper(II) ions is presented. The 2-HPB ligand featuring six pyridine moieties can bind to a metal center in a number of different ways. Herein, we discuss dicopper(II) complexes of 2-HPB that vary in coordination geometry. Thus, the four complexes obtained (1, 2, 3, and 4) adopt two different conformations, “(A,B)(C,D)-trans” (in 1 and 4) and “(A,B)(D,E)-trans” (in 2 and 3). The structure of the complex strongly depends on the solvent used (water, ethanol, and dimethylformamide (DMF)). In an aqueous solution, the formation of complexes 1 and 2 can be controlled by the choice of crystallization conditions. All the products were characterized by X-ray diffraction. The coordination preference of 2-HPB is evaluated in the context of hydrogen bonding in the solid state.


 Please wait while we load your content...
Please wait while we load your content...