On the kinetics of solvate formation through mechanochemistry†
Abstract
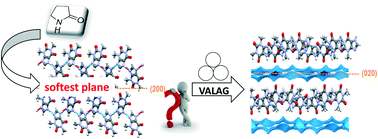
Theophylline : 2-pyrrolidone mono (1 : 1) and sesqui (2 : 3) solvates have been discovered through variable-amount liquid-assisted grinding (VALAG). The structures and stability of the solvates and the kinetics involved in their formation are investigated both experimentally and theoretically. Ex situ studies reveal a delayed appearance of the sesquisolvate and show that sesquisolvate formation occurs via the monosolvate rather than directly from pure theophylline. Theoretical calculations show that the obtained solvates are the thermodynamic products corresponding to the reactant ratio. The kinetics of the transformations was found to be related to the energy required to cleave the crystals through the softest planes. This was quantified by means of attachment energy calculations.
- This article is part of the themed collections: Introducing the CrystEngComm Advisory Board and their research, Editor’s Collection: Mechanochemistry and The Solid State of Pharmaceuticals


 Please wait while we load your content...
Please wait while we load your content...